What is SR-XRPD?
Introduction
X-Ray Powder Diffraction (XRPD) is a powerful experimental technique that uses the diffraction of X-rays on powder or microcrystalline samples for the structural characterization of materials [1,2].
XRPD allows for rapid, non-destructive in situ analysis of multi-component mixtures without the need for extensive sample preparation.
XRPD can be used to analyze quickly unknown materials and perform materials characterization in such fields as metallurgy, mineralogy, forensic science, archeology, condensed matter physics, and the biological and pharmaceutical sciences. In the field of pharmaceutical powders, XRPD is thus considered as the gold standard method for the identification of solid forms (i.e. polymorphs, solvates, hydrates, salts, co-crystals, amorphous) [3-5].
"SR-XRPD has thus caused powder diffraction methods to enter a whole new world of applications [6]."
In Synchrotron Radiation X-Ray Powder Diffraction (SR-XRPD) X-rays are generated by a synchrotron facility and are thus at least 5 orders of magnitude more intense than the best X-ray laboratory source. A tutorial on synchrotron radiation and SR-XRPD with exhaustive reference citations can be found on our Knowledge center.
The exceptional properties of synchrotron radiation applied to powder diffraction have thus caused powder diffraction methods to enter a whole new world of applications [6]. The high brilliance of the synchrotron radiation has drastically improved the structural characterization and level of detection of mixture components. When combining SR-XRPD with the new generation of solid-state ultra-fast detectors [7], Level of Detection (L.o.D.) smaller than 0.05% wt. are obtainable with acquisition times of a few minutes.
The speed of the measurement makes it possible to perform kinetic studies on structural changes during chemical reactions or under temperature and pressure ramps. The tunability and much wider range of available wavelengths than from laboratory X-ray sources has significantly increased the domain of applications of powder diffraction, for instance to biological molecules [8,9].


How does (SR)-XRPD work ?
A powder diffraction pattern is a fingerprint of the corresponding solid form. There is, in fact, a unique and direct relationship between the measured powder diffraction pattern and the structural order of the material under investigation [2,10,11].
Given a powder diffraction pattern of a given polycrystalline solid form:
○ The angular position of the diffraction peaks is uniquely defined by the size and dimension of the crystallographic unit cell.
○ The intensity ratio of the diffracted peak is uniquely defined by the location and type of the atoms in the unit cell.
○ The width and shape of the diffraction peaks is uniquely defined by the microstructural properties of the solid form under investigation (e.g. particle domain, strain, defects).
The more similar the structure or microstructure of two solid forms the less distinguishable their diffraction patterns (e.g. closer diffraction peaks, similar intensity ratios). The XRPD ability of discerning between two very similar polymorphic forms relies, therefore, on the quality of the XPRD measurement, which in turn depends on the X-ray source, diffractometer, detector and calibration procedure applied.
Subtle structural differences between two close polymorphs and/or low concentration of impurities can be totally hindered by XRPD measurements performed with moderately performing equipment.
Below as an example we show the 3D crystal structure of the High-Temperature metastable Form II of Carprofen [12] (courtesy of G. Bruni and F. Gozzo) and its corresponding High-Resolution (HR) synchrotron X-ray powder diffraction pattern acquired with Mythen II detector [7] in only 10 sec.
The structure solution of carprofen was not accessible with laboratory data nor its subtle polymorphism due to its isostructural character and configurational disorder. SR-XRPD made it possible.
XRPD can be seen as an objective analytical tool for structural and microstructural analyses of virtually any kind of material. Objective means that it does not require the recording of many reference diffraction patterns, as it is for example the case for Near Infra-Red Spectroscopy technique that needs complex spectral libraries and quantitative models to be constructed prior to qualitative and quantitative analyses.
A deeper discussion on the topic SR-XRPD vs NIR with extensive scientific references can be found on our Knowledge center page.
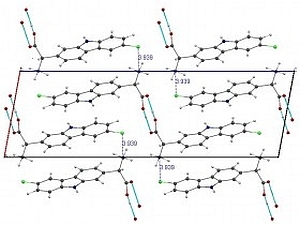
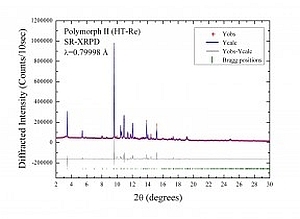
3D Crystal structure of Carprofen Synchrotron HR-XRPD pattern of Carprofen
Bibliography
- Bish, DL and Post, JE, editors. 1989. Modern Powder Diffraction. Reviews in Mineralogy, Vol. 20. Mineralogical Society of America
- Cullity, B. D. 1978. Elements of X-ray diffraction. 2nd ed. Addison-Wesley, Reading, Mass
- I. Ivanisevic, R. B. McClurg and P. J. Schields: Uses of X-Ray Powder Diffraction in the Pharmaceutical Industry, Pharmaceutical Sciences Encyclopedia: Drug Discovery, Development and Manufacturing, Ed. by Shayne C. Gad., p. 1-42 (2010).
- S. R. Byrn, S. Bates and I. Ivanisevic: Regulatory Aspects of X-ray Powder Diffraction, Part 1, American Pharmaceutical Review, p.55-59 (2005).
- D. Beckers: The power of X-ray analysis, Pharmaceutical Technology Europe (2010), p.29,30.
- M. Sakata, S. Aoyagi, T. Ogura & E. Nishibori (2007): Advanced Structural Analyses by Third Generation Synchrotron Radiation Powder Diffraction, AIP Conference Proceedings, Vol. 879, pp. 1829-1832 (2007).
- Bergamaschi, A.; Cervellino, A.; Dinapoli, R.; Gozzo, F.; Henrich, B.; Johnson, I.; Kraft, P.; Mozzanica, A.; Schmitt, B.; Shi, X.: The MYTHEN detector for X-ray powder diffraction experiments at the Swiss Light Source. J. Synchrotron Rad. 17 (2010) 653–668.
- R.B. Von Dreele, P.W. Stephens, G.D. Smith, and R.H. Blessing: The First Protein Crystal Structure Determined from X-ray Powder Diffraction Data: a Variant of T3R3 Human Insulin Zinc Complex Produced by Grinding,Acta Cryst. D 56, 1549-53 (2000).
- Margiolaki, I., Wright, J. P., Fitch, A. N., Fox, G. C. & Von Dreele, R. B.: Synchrotron X-ray powder diffraction study of hexagonal turkey egg-white lysozyme, Acta Cryst. D61, 423–432 (2005). See also: Margiolaki, I. & Wright, J. P.: Powder crystallography on macromolecules, Acta Cryst. A64, 169–180 (2008).
- Fundamentals of Crystallography,C. Giacovazzo Ed., International Union of Crystallography, Oxford Science Publications, Third Edition (2011).
- The basics of Crystallography and Diffraction (Third edition, 2010), Christopher Hammond, IUCr, Oxford Science Publications; ISBN 978-0-19-954645-9. See also: X-Ray Structure Determination, A practical Guide, George H. Stout and Lyle H. Jensen, Wiley Interscience.
- Bruni G, Gozzo F, Capsoni D, Bini M, Macchi P, Simoncic P, Berbenni V., Milanese C., Girella A., Ferrari S. and Marini A., Thermal, Spectroscopic, and Ab Initio Structural Characterization of Carprofen Polymorphs, J. Pharm. Sciences 100(6), 2321 (2011).
- Brunelli, M., Wright, J. P., Vaughan, G. B. M., Mora, A. J. & Fitch, A. N.: Solving Larger Molecular Crystal Structures from Powder Diffraction Data by Exploiting Anisotropic Thermal Expansion. Angew. Chem. (2003) 115, 2075–2078.
- T. Wessels, Ch. Baerlocher and L.B. McCusker: Single-crystal-like diffraction data from polycrystalline materials, Science (1999), 284, 477-479.
- Shankland, K., David, W. I. F., Csoka, T. & McBride, L.: Structure solution of Ibuprofen from powder diffraction data by the application of a genetic algorithm combined with prior conformational analysis, Intl. J. Pharmaceut. (1998) 165, 117–126.
- A. Altomare, C. Cuocci, C. Giacovazzo, A. Moliterni and R. Rizzi: The dual-space resolution bias correction algorithm: applications to powder data, J. Appl. Cryst. (2010). 43, 798-804.
- G.Oszlanyi and A. Suto: The charge flipping algorithm, Acta Cryst. (2008). A64, 123–134 and references herein.
- Boccaleri, E., Carniato, F., Croce, G., Viterbo, D., van Beek, W., Emerich H. and Milanesio, M., In-situ simultaneous Raman/high-resolution X-ray powder diffraction study of transformations occurring in materials at non-ambient conditions, J. Appl. Cryst., 2007, 40, 684-693.
- Scarlett N.V.Y. and Madsen I. C., Quantification of phases with partial or no known crystal structures, Powder Diffraction (2006) 21, 278-284
- Giannini C., Guagliardi A. and Millini R., Quantitative phase analysis by combining the Rietveld and the whole-pattern decomposition methods, J. Appl. Cryst., 2002, 35, 481-490.
- Scardi, P.; Leoni, M.: Line profile analysis: pattern modeling versus profile fitting, J. Appl. Cryst. 39 (2006) 24–31. Scardi, P.; Leoni, M.: Whole Powder Pattern Modelling, Acta Crystall. A58 (2002) 190–200
- Local structure from total scattering and atomic pair distribution function (PDF) analysis, In Powder diffraction: theory and practice, (Royal Society of Chemistry, London England, 2008), Robert E. Dinnebier and Simon J. L. Billinge, Eds., pp. 464 – 493.
- Neder R. B. And Korsunskiy V. I., Structure of nanoparticles from powder diffraction data using the pair distribution function, 2005 J. Phys.: Condens. Matter 17 S125
- A. Cervellino, C. Giannini, A. Guagliardi, Determination of nanoparticle, size distribution and lattice parameter from x-ray data for monoatomic materials with f.c.c. cubic unit cell, J. Appl. Cryst. 36, 1148-1158 (2003).
- P.W. Stephens, D.E. Cox, and A.N. Fitch, Synchrotron Radiation Powder Diffraction in Structure Determination by Powder Diffraction, pp. 49-87, edited by W.I.F. David, K.
Shankland, L.B. McCusker, and C. Baerlocher, (Oxford University Press, 2002) - Joel Bernstein: Polymorphism in Molecular Crystals, IUCr Monographs on Crystallography (2002), Oxford Science Publications.
- Polymorphism in Pharmaceutical Solids, Ed. By Harry G. Brittain, Drugs and The Pharmaceutical Sciences, Vol. 192
- Tremayne, M.: The impact of powder diffraction on the structural study of organic materials., Philosophical Transactions of the Royal Society of London Series A: Chemistry and Life Sciences Triennial Issue, 362: 2691 (2004).
- Law D, Schmitt EA, Marsh KC, Everitt EA,Wang W, Fort JJ, Krill SL, Qiu Y. Ritonavir-PEG 8000 amorphous solid dispersions: in vitro and in vivo evaluations. J. Pharm. Sci. 2004; 93 (3): 563–567.
- Bruni G., Berbenni V., Milanese C., Girella A., Cardini A., Lanfranconi S. and Marini A., Determination of the nateglinide polymorphic purity through DSC, J. Pharm. and Biomed. Anal. 54 (2011) 1196-1199.
- Aaltonen J., Alleso M., Mirza S., Koradia V., Gordon K.C and Rantanen J., Solid form screening – A review. European Journal of Pharmaceutics and Biopharmaceutics 71 (2009), 23-37.
- Srivastava D., The Food and Drug Administration and Patent Law at a Crossroads: The Listing of Polymorph Patents as a Barrier to Generic Drug Entry. Food and Drug Law Journal, Vol. 59, No.2 (2004), 339-354.
- Grabowski H. G., Kyle M., Mortimer R., Long G. & Kirson N., Evolving Brand-name And Generic Drug Competition May Warrant A Revision Of The Hatch-Waxman Act. Health Affairs, 30, no.11 (2011):2157-2166.
- Rakowski W. A and Mazzochi D. M., The case of disappearing polymorph: 'Inherent anticipation' and the impact of Smithkline Beecham Corp. v Apotex Corp. (Paxil®) on patent validity and infringement by inevitable conversion'. Journal of Generic Medicine, Vol.1, No.2 (2006):131-139.
- Cabri W., Ghetti P., Pozzi G. and Alpegiani M., Polymorphisms and Patent, Market, and Legal Battles: Cefdinir Case Study. Organic Process Research & Development 2007, 11, 64-72.
- Bauer J, Spanton S, Henry R, Quick J, Dziki W, Porter W, Morris J., Ritonavir: an extraordinary example of conformational polymorphism, Pharm Res. 2001 Jun;18(6):859-66.


